MiGenTra Aims to Ensure Equitable Access to Essential Medicines to Overcome Healthcare Inequality
MiGenTra strives to establish equitable access to high-quality biologics at affordable prices for Egypt, the Middle East, and Africa (MEA) while simultaneously strengthening regional and local manufacturing capacities. The company thus contributes to the Global Health Initiative and efforts by the World Health Organization (WHO) and the International Finance Corporation (IFC) to address urgent, unmet medical needs.
To overcome the healthcare inequality our vision is to transform healthcare in Africa & Middle East (MEA) by our mission to provide quality and affordable biological medicines to more patients in the MEA region.
Company
- Founded 2021
- Based in Berlin, Germany
- Subsidiary of ProBioGen AG
(Germany) and Minapharm
Pharmaceuticals SE (Egypt)
Holistic Approach to Cover the Needs Towards Better Healthcare in Africa
The MEA region has long dealt with the repercussions of inadequate access to critical and lifesaving medicinal products. Fragile healthcare systems, expensive biologics, and limited regional manufacturing capacity has compounded the issue. This lack of access to affordable drugs and treatments could jeopardize the economic growth of these nations.
With patents on biologic drugs due to expire, now is the time to seize the opportunity to develop affordable alternatives. However, time is of the essence. Data shows that only the first four products to market will garner enough of the market share to offset R&D expenses and secure future development. MiGenTra, guided by the WHO and IFC global health initiative on affordable medicine and treatment access, is poised to transform healthcare in the MEA region.
MiGenTra’s disruptive go-to-market business model and strategic partnerships will position MEA as a new global hub for healthcare product manufacturing.
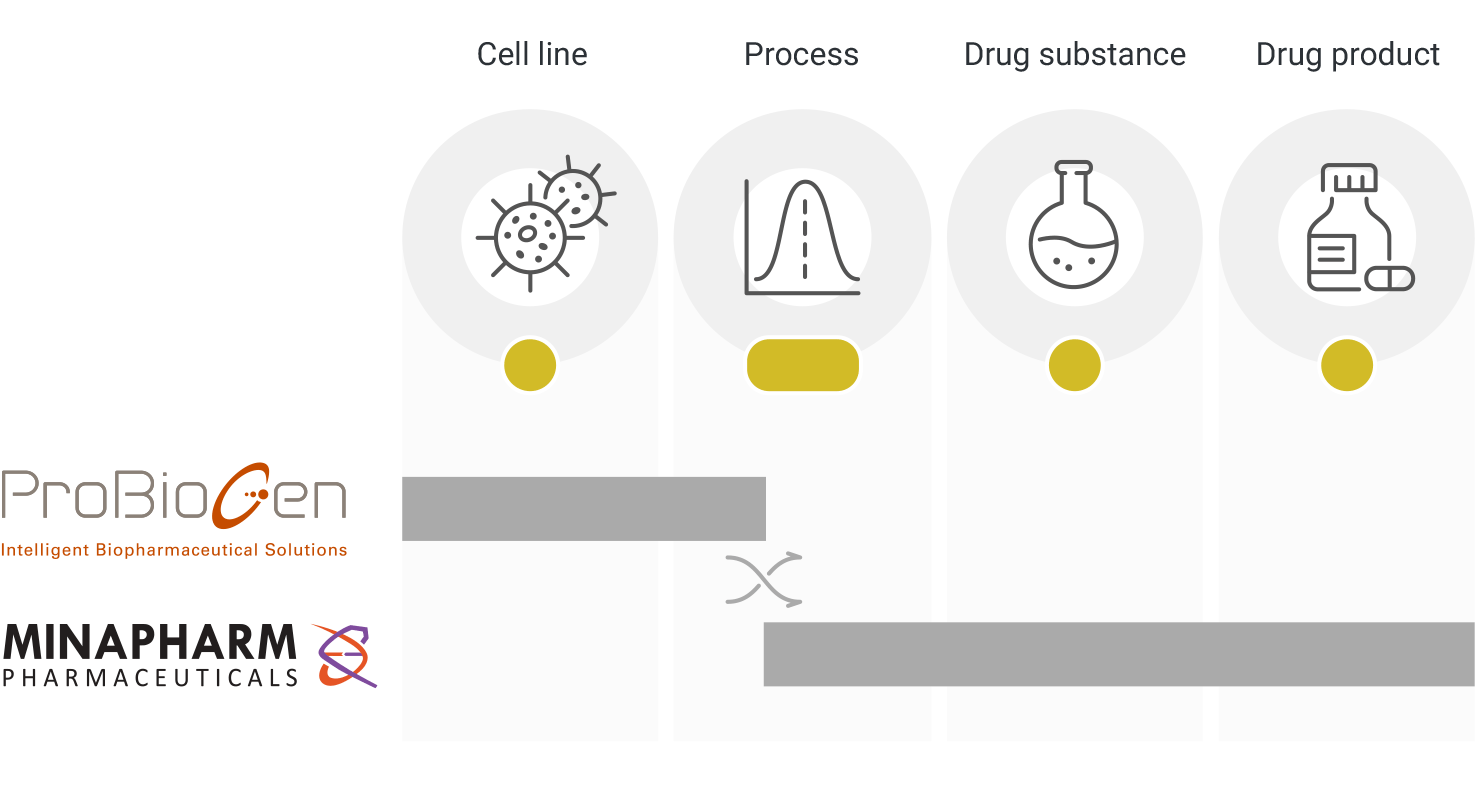
Thanks to Minapharm’s continued regional success over global players and its solid track record in biological drug development and ProBioGen’s cutting-edge technologies and unparalleled knowledge in the development and manufacture of active ingredients and vaccines, MiGenTra is poised to succeed at closing the gap to equitable medical access.
TREAT Strategy

Therapies: Evaluating large untapped market opportunities for healthcare products already in development.

REAch: Building collaborations with biopharmaceutical partners for commercialization and branding.

Transformation: Selecting new molecules as well as early in-house development of wave 3 molecules.
Leveraging the Best of Two Worlds
With its founding partners headquartered in Berlin, Germany, and Cairo, Egypt, MiGenTra is uniquely positioned to reap the benefits of a solid foothold in the MEA region and a significant European customer base.
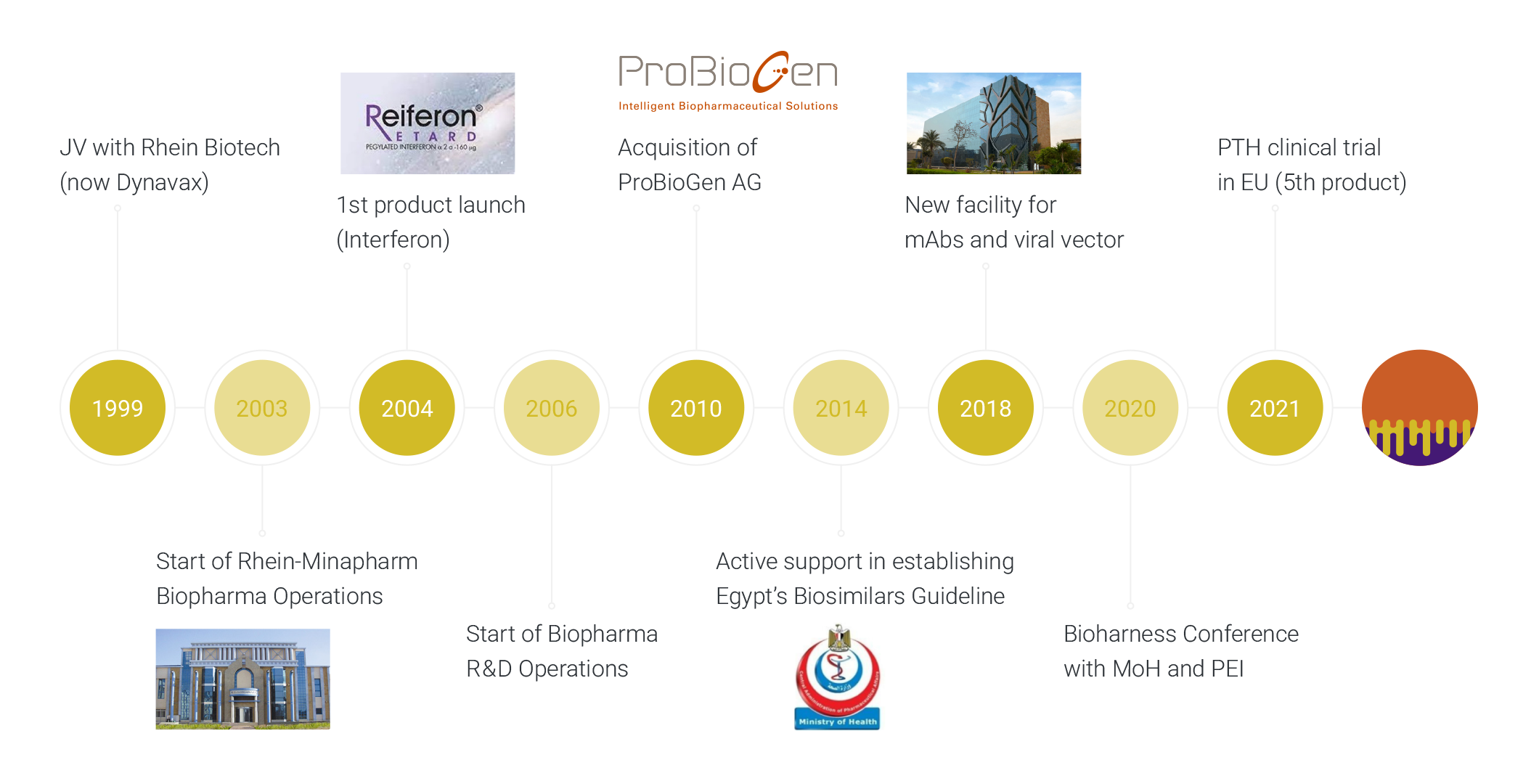
Incorporated in 2021 by ProBioGen (internationally known CMDO) and Minapharm Pharmaceuticals (only gene-to-market biopharmaceutical company in Africa & the Middle East), MiGenTra fuses the scientific, manufacturing, and commercial expertise of its parent companies towards the end-to-end development and commercialization of high quality affordable biologic medicines in the field of Biosimilars, Cell- and Gene Therapies and Vaccines to serve the needs of patients across Africa and the Middle East. The company is based in Berlin, with management both in Berlin and Cairo.
Innovation Made Affordable, Commercial Ready Now
- Uniquely positioned to serve Africa with quality biologic therapies at affordable cost
- Current network and planned geographic expansion to cover most of Africa & the Middle-East.
- Presence in 12 markets via 18 partners; active expansion ongoing through new product registrations
Specialised in the Development and Manufacturing of Complex Therapeutic Proteins
Specialized in the development and manufacturing of complex therapeutic proteins, ProBioGen is a Contract Development and Manufacturing Organization (CDMO), a technology provider located in Berlin, Germany, and a subsidiary of Minapharm Pharmaceuticals in Egypt. With over 25 years of experience, ProBioGen is a sought-after partner along the entire drug development value chain up to clinical phase III.
The company’s over 300 employees include internationally acclaimed scientists specialized in biopharmaceutical cell development and technology, protein chemistry, and molecular biology. ProBioGen also has extensive knowledge and expertise in the development of viral vector platforms and gene-based therapies. ProBioGen also provides process development services and manufacturing technologies for viral vaccines.
With its intelligent technologies and state-of-the-art platforms, ProBioGen generates biologics with optimized properties. With a comprehensive quality management system, the company ensures compliance with international ISO and GMP standards (EMA/FDA). In order to meet high customer demand, the company is expanding its current headquarters to increase production capacity by adding an additional 1000 L single-use bioreactor soon.
- Berlin, Germany
- Internationally renowned CDMO and technology provider
- Cell line engineering, process development, GMP manufacturing, and analytics of biopharmaceuticals
- 25+ years of experience
- 250+ employees
- 2,000 L bioreactor capacity
- Cairo, Egypt
- Sole gene-to-market biopharmaceutical company in Africa and the Middle East
- Integrated R&D department operated by highly skilled scientists and cutting edge 15,000 m2 end-to-end biomanufacturing facility
- 20+ years of track record in biotech
- 1,500+ employees
- 11,000 L bioreactor capacity
State-of-the-Art Pharmaceutical and Biopharmaceutical Research Technologies Made Affordable
Minapharm has dedicated 50 years to ensuring patients in developing countries also benefit from state-of-the-art pharmaceutical and biopharmaceutical research technologies. As the sole gene-to-market biopharmaceutical company in the MEA region, Minapharm is positioned to play a vital role in the development and manufacture of Biosimilars, Cell and Gene Therapies (CGT), and vaccines for the MEA region.
With over 1400 employees, the company maintains a diverse product portfolio that covers an extensive range of medical products, from small molecules to complex bioengineering proteins. In its effort to direct the region’s focus towards biologicals, the company has already set up a second manufacturing facility to help meet its short and medium-term biotechnology and gene therapy business goals.
Together with its wholly owned Berlin-based subsidiary, ProBioGen AG, Minapharm has established an integrated business model making it the only gene-to-market biopharmaceutical company in the region to date.
Leadership Team
Dedicated to overcoming disparity on the African continent and beyond by bringing healthcare transforming products and services to market MiGenTra is driven by a diversified and highly experienced management team with in-depth knowledge of the region.

Dr. Lutz Hilbrich, Senator at Senate of Economy Europe, holds a Medical Degree from the Julius-Maximilians-University, Würzburg, Germany and an MBA from Stern School of Business at NYU, USA. Before joining ProBioGen, he was the Head of the Biosimilars Platform China for Sanofi and second-in-command for the Biosimilars Business Unit at Boehriger Ingelheim GmbH, Germany. He is experienced in CMC, pre-clinical, and clinical development but also in business development and market access strategies. Besides serving on a couple of organizational committees for international conferences on biologics and biosimilars, he is on the Biosimilar Advisory Board of the American Conference Institute (ACI). Since June 2020, he has been CEO of ProBioGen. Under his leadership, the idea of spinning out a product development company materialized with the incorporation of MiGenTra GmbH in April 2021.

Dr. Shaheer Bardissi is an executive board member at Minapharm pharmaceuticals and advisory board member at MiGenTra GmbH. Shaheer has been assigned the responsibility of aiding the steering of the growth strategy of the Minapharm group in the field of immuno-oncology, vaccines for infectious diseases, and gene and cell-based therapies in collaboration with its global subsidiaries. Shaheer previously worked at BioNTech SE and contributed to the successful development of immunotherapeutic technologies in the field of cell and gene therapy. Shaheer obtained his PhD from Johannes Gutenberg University, Mainz, Germany, with a focus on gene-based immunotherapy. Prior to that, he completed his Masters of Science in Molecular Medicine at University College London, Bachelors of Science in Biomedical Sciences from King's college London, and also holds a Masters of Science in quantitative management sciences and healthcare data analytics from Duke University, North Carolina, USA.

Frédéric joined MiGenTra in February 2022 bringing more than 20 years’ experience in functions spanning from strategic marketing to strategic planning, portfolio management and business development & licencing. Prior to joining MiGenTra, Frederic served as Head Strategy & Portfolio Management for Fresenius Kabi SwissBioSim and Head Biosimilars for Sanofi’s Global Diabetes Division. Frederic holds a MSc in Organic Chemistry, a MSc in Chemical Engineering, and an MBA.

Vice President Finance & Administration at ProBioGen since 2015 and Chief Financial Officer since 2021. Andrea Hauptmann has been responsible for financial reporting, controlling, funding and cash management, risk management, human resources and administration since 2011. Before joining ProBioGen in 2009, she held a number of key financial management positions in the service and real estate sector. She was granted power of attorney in 2009. Andrea Hauptmann holds a diploma in from Technical University (TH) of Zittau, Germany.
Advisory Board
As we have embarked on our mission to develop and commercialize quality and affordable biologic medicines to transform healthcare in Africa, our international Advisory Board with highly distinguished leaders in science and entrepreneurship will adviser and provide guidance to support future product development and regulatory, market excess and export strategies.
Careers
Be Part of the Transformational Change Across Healthcare
Are you interested in being part of a team committed to sustainable change in global healthcare and providing affordable and critical medical treatments? Then learn more about our career opportunities and find out how you can make a lasting impact.