Ensuring Affordable Access to High-Quality Biologics Across the MEA Region
MiGenTra fuses the distinct strengths and capabilities of its two founding companies, ProBioGen and Minapharm, to bring a unique and comprehensive offer to market. With its strong foothold in the MEA region (Egypt, the Middle East and Africa) and successful track record with biosimilars, Minapharm is firmly positioned ahead of the local competition. ProBioGen has garnered international recognition for its team of scientists and their unparalleled knowledge of biopharmaceutical cell development and technology.
Past collaborations between the two companies on synergy projects have yielded successful results, including Minapharm’s development of mAb biosimilars. This new joint venture will propel the development and commercialization of biosimilars, CGT, and vaccines in an underrepresented region with largely overlooked medical needs.
Combining Minapharm's extensive experience with biosimilars and ProBioGen's expertise in cell and gene-based therapeutic (CGT) approaches and viral vector platforms for vaccine development, MiGenTra is ideally positioned to provide patients with inexpensive and quality care and boost the region's drug manufacturing capabilities.
Biosimilars – from Cell Line Development to Market
Biosimilars are molecularly complex, near copies of biological drugs that can be affordably manufactured and therefore more widely distributed. With biologic drug patents set to expire, the race to introduce affordable biosimilars to the African continent is quickly gaining momentum.
The biosimilar market is forecasted to have a compound annual growth rate of 32 percent between 2020 and 2026. Though the market is only emerging, Africa’s growing population, the exorbitant prices for biologics, and unmet medical needs in the region are the perfect breeding ground for a flourishing biosimilar industry. The growth potential has already attracted the interest of big players in the biosimilar industry, many of whom are now attempting to collaborate with local partners.
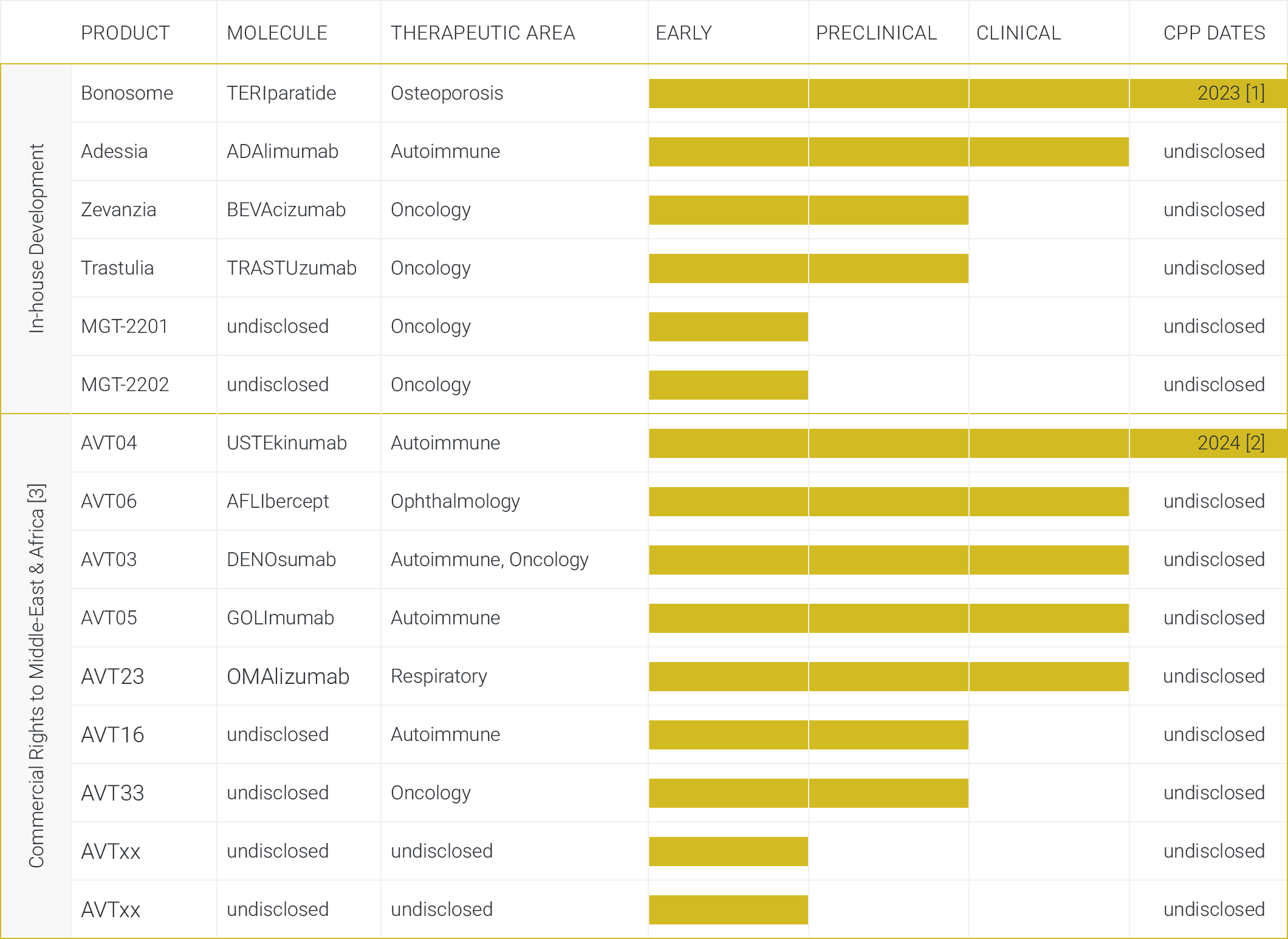
MiGenTra already has a head start. With Minapharm’s in-house biosimilar development of anti TNF alpha, HER2, and VEGF as well as its established position in the region, MiGenTra is ready to seize on commercialization opportunities by establishing strategic partnerships to raise brand awareness and boost market access.
Key Benefits Biosimilars
- Proven track record (anti TNF alpha, HER2, and VEGF)
- Local development and manufacture
- Established position in the MEA region
- Unmet emerging market opportunities
- Strategic partnerships to raise brand awareness and boost market access
Key Benefits of Cell & Gene Therapies (CGT)
- First-class expertise in cell line and process development
- Egypt-based manufacturing facility
- In-licensing and obtaining rights for the MEA region
- Unmet emerging market opportunities
At the Forefront of CGT Development and Manufacturing
Cell and gene therapy is at the frontier of ground-breaking treatments for rare and often incurable diseases. By correcting errors in the genome, nucleic acid and cell-based therapies can cure the underlying genetic cause of diseases as well as redirect the patient's immune system to treat previously untreatable diseases such as cancer.
There are currently 2500 ongoing CGT clinical trials worldwide, none of which are being conducted in Africa. The primary obstacles have been a lack of regional manufacturing capacity and regulatory transparency. As a result, such therapies have not yet been approved in the region. Nevertheless, a predicted compound annual growth rate of 30 percent by 2025 for the CGT industry has sparked a growing interest in the region.
MiGenTra is well positioned to alleviate significant barriers to CGT manufacture and development in the region. The company produces its own CGT products for the MEA region and uses its proprietary technologies to help secure new contracts. With ProBioGen on board to help procure good manufacturing process (GMP) certification, the company’s new Cairo viral vector manufacturing facility is an attractive option for global biopharmaceutical partners.
A Local End-to-End Manufacturing Hub for Life-Saving Vaccines
The COVID-19 pandemic reminded the world of inequalities regarding the availability of vaccines, diagnostics, and therapeutics, with Low and Middle Income Countries (LMICs) at the end of the line. The absence of local end-to-end vaccine manufacturing contributes significantly to this problem.
ProBioGen has generated scalable and flexible production processes for human vaccines that circumvent the challenges of supply, variable performance and increasing costs for source material and extensive safety testing. Building on ProBioGen’s knowledge in process development and Minapharm’s decades of experience in providing innovative and affordable biologics, MiGenTra’s access to a cutting-edge biologics manufacturing facility in Cairo makes it perfectly positioned for the local and end-to-end manufacture of life-saving vaccines for the African continent and other emerging countries at large.
MiGenTra develops its own second-generation vaccines and also partners with global biopharma acting as a local manufacturing hub and gateway to emerging countries, its aim being to provide them with access to life-saving vaccines.
Key Benefits Vaccines
- First-class expertise in process development
- Scalable and flexible production processes
- Egypt-based manufacturing facility
- Unmet emerging market opportunities